Alzheimer’s is a deadly disease, claiming 121,499 lives in the U.S., making it the sixth-leading cause of death in the country. Alzheimer’s is a neurodegenerative disease caused by the degeneration of neurons in brain areas related to cognition. The initial symptoms often include memory, language, and thinking difficulties. Daily living can become extremely difficult for individuals with Alzheimer’s, and they may also have changes in mood, personality, and behavior, which vary among individuals.
TDP-43 in Cognitive Abilities
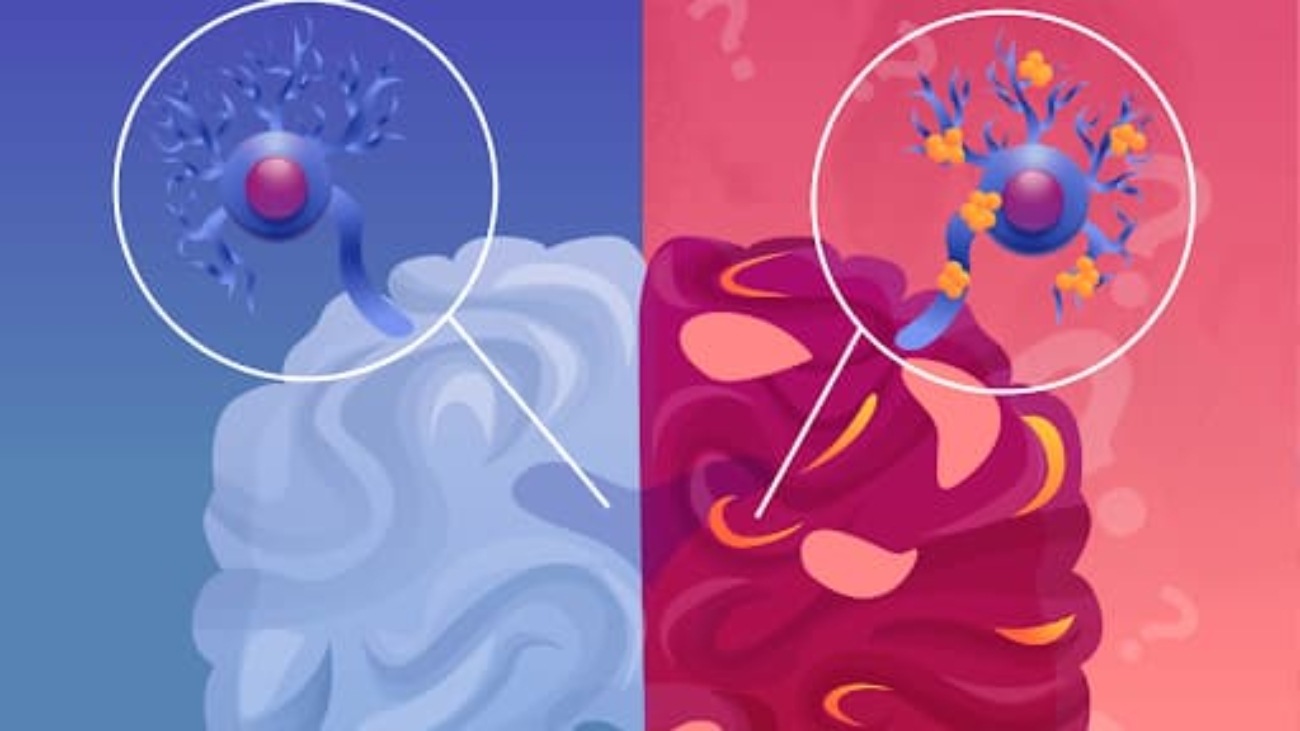
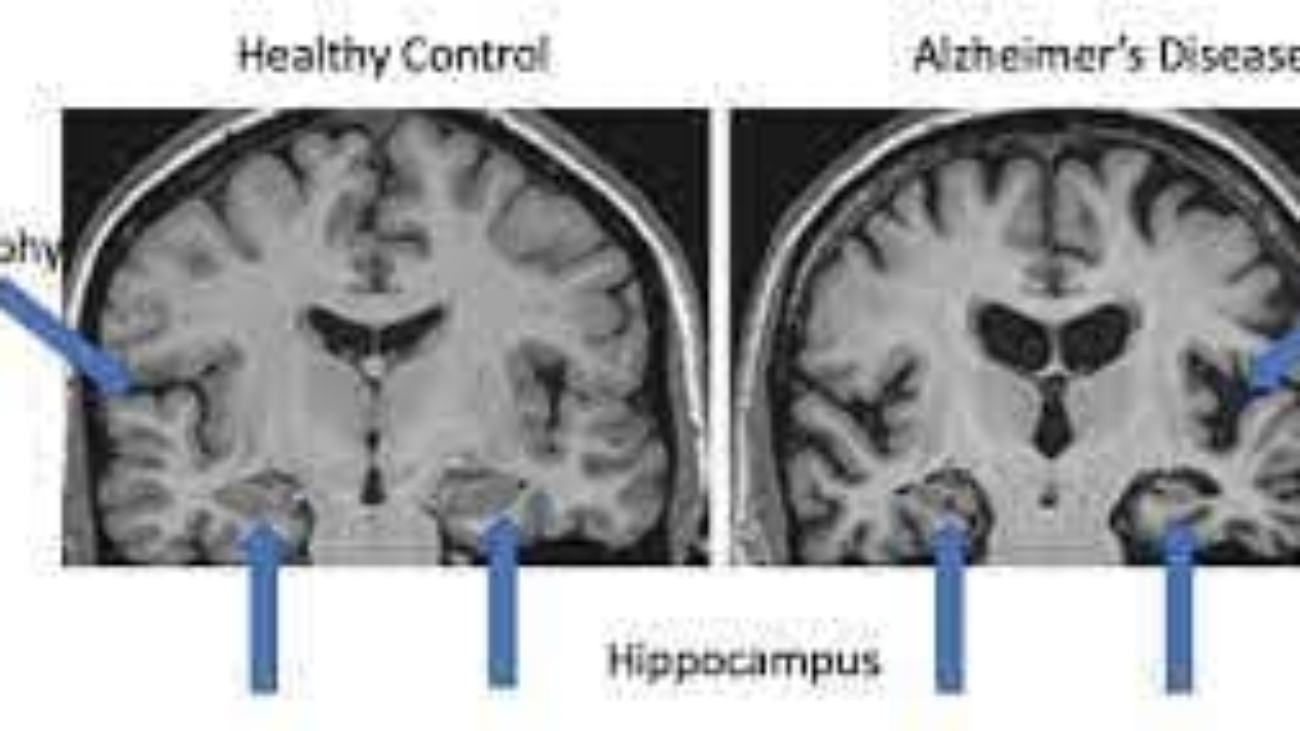
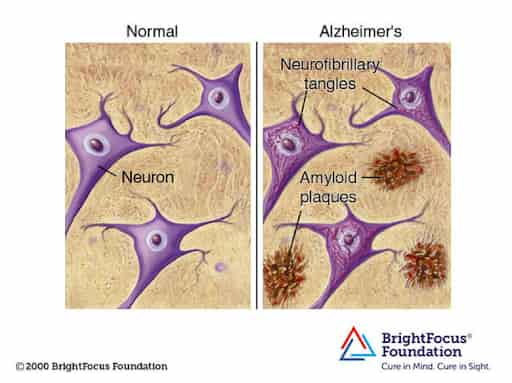
At the molecular level, the accumulation of beta-amyloid proteins outside the neurons and tau proteins inside neurons are significant characteristics of the disease. This accumulation can lead to inflammation and brain tissue atrophy, atrophy contributing to neuronal death. Dementia, a condition commonly found in Alzheimer’s patients, involves a decline in cognitive abilities such as memory loss, difficulty in reasoning, and impaired communication skills. It significantly affects a person’s ability to perform daily tasks independently.
Molecular composition of TDP-43
As toxic beta-amyloid proteins and tau proteins accumulate, microglia, specialized cells in the brain, activate (feel free to check out our other article on microglia!) to clear away debris from dying cells. This process, however, can lead to inflammation when microglia struggle to keep up. A crucial protein involved in Alzheimer’s is TDP-43, a transactive response DNA binding protein of 43 kDa, which is encoded by the TARDBP gene. TDP-43, in its normal state, is involved with RNA processing, brain stability, and gene regulation. When TDP-43 is phosphorylated and truncated (shortened and phosphate groups are added), it becomes linked to amyotrophic lateral sclerosis. In Alzheimer’s, TDP-43 forms neurofibrillary tangles, contributing to the disease’s progression and cognitive decline.TDP-43, a nuclear ribonucleoprotein, influences mRNA levels, including tau expression. Dysregulation of atu leads to its aggregation, a hallmark of Alzheimer’s. Mutations affecting TDP-43’s nuclear localization sequence cause mislocalization, and new drugs are being created to target this sequence.
Depletion of TDP-43 in the nucleus results in cytoplasmic aggregation, which is what causes Alzheimer’s. Understanding TDP-43 may offer insights into potential Alzheimer’s treatments.
Sources:
- https://molecularneurodegeneration.biomedcentral.com/articles/10.1186/s13024-021-00503-x
- https://alz-journals.onlinelibrary.wiley.com/doi/10.1002/alz.13016#:~:text=This%20number%20could%20grow%20to,death%20in%20the%20United%20States.